Education
- B.S., Chemistry, University of Colombo, 2009
- PhD, Inorganic Chemistry, University of Kansas, 2015
- Post-Doctoral Associate, Johns Hopkins University, 2015-2018
Research Areas
- Inorganic Chemistry
- Biochemistry
Research
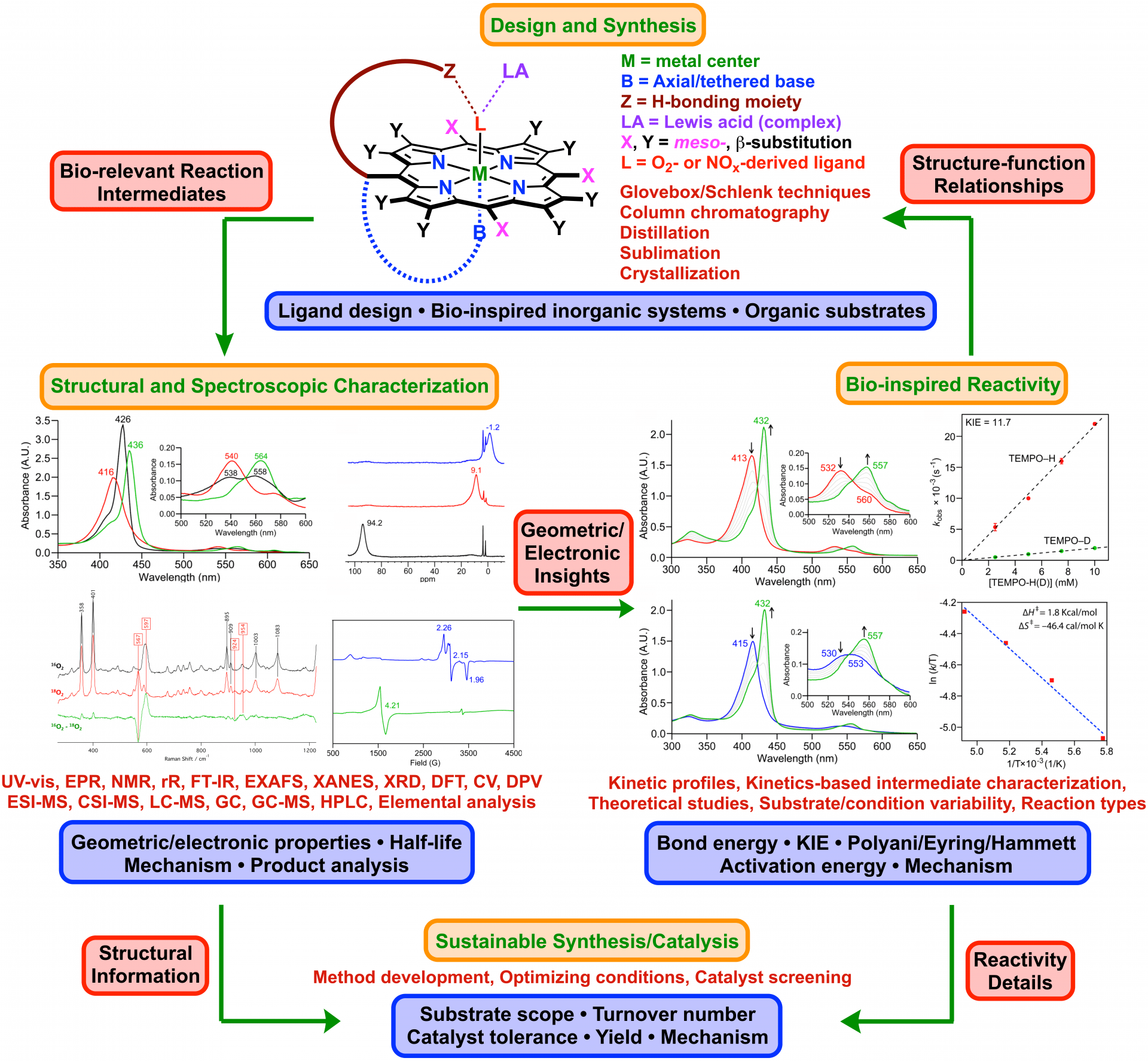
We design and generate bioinspired synthetic model complexes of heme enzymes that activate small molecules/ions such as dioxygen (O2) and/or nitrogen oxides (NOx’s; e.g., NO, N2O, NO2–, NO3–) in biology. Detailed investigations into these mimics often shed crucial light on analogous enzymatic systems (structure-function relationships, mechanism, thermodynamic and kinetic properties etc.), and hence can be powerful mechanistic probes for some of the most understudied heme enzymes, especially those that are rapidly emerging drug targets against a vast variety of neurodegenerative and autoimmune conditions, as well as a broad array of cancers.
Student and postdoctoral researchers engaged in these research attempts will progress to adepts in synthetic, spectroscopic, and structural approaches for tackling mechanistic ambiguities, with a sound knowledge of biological, inorganic, organic and physical chemistries. We actively collaborate with multiple research groups that bring in various expertise, and routinely work with several on-campus UAB centers such as the UAB O’Neal Comprehensive Cancer Center (CCC), UAB Center for Free Radical Biology (CFRB), and UAB Center for Nanoscale Materials and Biointegration (CNMB).
1. Modeling Reactivity Landscapes of Mid-Valent Heme-oxygen (i.e., Heme-Superoxo/-Peroxo/-Hydroperoxo) Intermediates
Unlike their high-valent counterparts, chemistries involving mid-valent heme-oxygen intermediates are severely understudied despite their implications as active and/or important oxidants in a wide variety of heme systems in biology. Mid-valent intermediate of synthetic heme systems have long been categorized as sluggish oxidants, albeit our recent work suggests that meticulously designed model systems can be highly competent, versatile oxidants that can carry out unique reactivities that closely parallel their biological analogs.
2. Understanding the Nitrogen Oxide (NOx/y) Activation and Interconversion Chemistries Involving Heme Centers
Significant ambiguities exist surrounding the precise pathways that are activated under physiological oxidative stress, nitrative/nitrosative stress, or both. Biological heme centers have, in some instances, been implicated in these transformations, although their exact role(s) or their significance in human health and diseases remains elusive. We use synthetic heme mimics to probe these possibilities tied to NOx/y interconversion pathways and to gain insight into their potential relevance in targeted therapy.
3. Bioinspired Synthetic Chemistry using Heme Centers
Heme enzymes mediate a plethora of organic transformations in biology under environmentally and economically benign reaction conditions. Some of these transformations generate extremely precious organic motifs that are often difficult to obtain with traditional organic chemistry synthetic routes, yet are of pivotal value as small molecule building blocks. We utilize our synthetic heme model compounds as bioinspired reaction centers in modeling such reactivities that allows the investigation of their utility in next generation synthetic attempts that are cheaper, greener, and highly efficient compared to current methodologies. An added benefit of this approach is that we also get to learn mechanistic details pertaining to biological heme systems that can be implemented in the rational design of novel therapeutic agents targeting those heme enzymes.
Selected Publications
Mondal, P.; Ishigami, I.; Yeh, S.-R.; Wijeratne, G. B.* The Role of Heme Peroxo Oxidants in the Rational Mechanistic Modeling of Nitric Oxide Synthase: Characterization of Key Intermediates and Elucidation of the Mechanism. Angew. Chem. Int. Ed. 2022, 61, e202215829.
Mondal, P.; Rajapakse, S.; Wijeratne, G. B.* Following Nature’s Footprint: Mimicking the High-Valent Heme-Oxo Mediated Indole Monooxygenation Reaction Landscape of Heme Enzymes J. Am. Chem. Soc. 2022, 144, 3843–3854
Mondal, P.; Tolbert, G. T.; Wijeratne, G. B.* Bio-inspired Nitrogen Oxide (NOx) Interconversion Reactivities of Synthetic Heme Compound-I and Compound-II Intermediates J. Inorg. Biochem. 2022, 226, 111633– 111645
Mondal, P.; Ishigami, I.; Gérard, E. F.; Lim, E.; Yeh, S.-R.; de Visser, S. P.*; Wijeratne, G. B.* Proton-coupled Electron Transfer Reactivities of Electronically Divergent Heme Superoxide Intermediates: A Kinetic, Thermodynamic, and Theoretical Study Chem. Sci. 2021, 12, 8872-8883
Mondal, P.; Wijeratne, G. B.* Modeling Tryptophan/Indoleamine 2,3-Dioxygenase with Heme Superoxide Mimics: Is Ferryl the Key Intermediate? J. Am. Chem. Soc. 2020, 142, 1846
Wijeratne, G. B.*; Bhadra, M.; Siegler, M. A.; Karlin, K. D.* Copper(I) Complex Mediated Nitric Oxide Reductive Coupling: Ligand Hydrogen Bonding Derived Proton Transfer Promotes N2O(g) Release J. Am. Chem. Soc. 2019, 141, 17962-17967